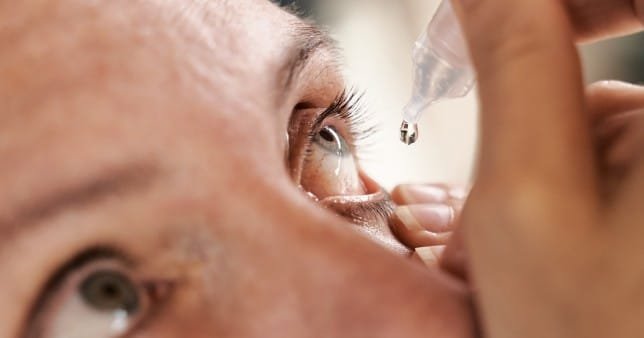
In the US, tainted eyedrops have caused the deaths of three persons and the need for eyeball removal in four more.
According to the Centers for Disease Control and Prevention (CDC), 68 or more persons have been affected by over-the-counter eyedrops made by EzriCare and Delsam Phama throughout 16 states.
On March 14, the count was accurate.
The CDC reported that “three persons have died, eight have experienced vision loss, and four have had their eyeball surgically removed.”
37 of the patients received care at four different healthcare facility groups.
The majority of patients reported using artificial tears. The drops have caused a strain of Pseudomonas aeruginosa that ‘had never been reported in the United States prior to this outbreak’, according to the CDC.
It is ‘a rare strain of extensively drug-resistant’ Pseudomonas aeruginosa, the agency stated.
The outbreak is associated with several types of infections including of the eye. Eye infection symptoms include redness on the eye or eyelid and yellow, green, or clear discharge from the eye. Patients can also experience eye pain or discomfort, feeling that something is in the eye, blurry vision and increased sensitivity to light.
People who used the products and are not experiencing symptoms do not need to get tested.
Health officials are testing unopened bottles of EzriCare Artifical Tears to determine if the contamination took place in the manufacturing phase.
Patients and providers are advised to stop using EzriCare or Delsam Pharma artificial tears until the CDC and Food and Drug Administration release more information and guidance.